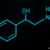
Midazolam is a benzodiazepine dosed at 0.2-0.3 mg/kg IV; the onset of action is 60-90 seconds, and the duration of action is approximately 15-30 minutes.1 Midazolam has direct negative effects on systemic vascular resistance and the myocardium. In a prospective study comparing midazolam versus etomidate, Choi and colleagues observed a 10% reduction in mean systolic blood pressure in patients receiving midazolam.21 The poor side effect profile and slow onset of action make midazolam an unfavorable induction agent.
Explore This Issue
ACEP News: Vol 29 – No 09 – September 2010Paralysis
Paralytics are divided into two NMBA classes: noncompetitive and nondepolarizing. Succinylcholine (SCh) is a noncompetitive NMBA dosed at 1.5-2 mg/kg IV or 4 mg/kg IM; the onset of action is 45 seconds, and the duration of action is approximately 6-8 minutes. SCh is the most popular paralytic among emergency physicians, as it boasts a short onset and relatively quick return of airway reflexes.
A large disadvantage of SCh is the potential for life-threatening hyperkalemia in at-risk patient populations. Traditionally, patients with renal failure have been listed in this at-risk cohort. However, multiple case series and meta-analyses yielded no reports of dysrhythmias or adverse events in the setting of SCh use in renal failure. Also, the mean increase in potassium was approximately 0.5 meq/L.22,23,24 While not recommended, inadvertent SCh administration in the setting of renal failure appears to be safe. Fatal hyperkalemia is seen in receptor upregulation (e.g., burns, crush injuries, upper or lower motor neuron injuries, and prolonged ICU stays) and myopathies (e.g., muscular dystrophy). SCh use in myopathies causes a significantly higher mortality (30%) than does its use in receptor upregulation (11%).25
SCh has also been associated with bradycardia, especially in repeat dosing. This bradycardia has not been shown to be prevented by prophylactic atropine; however, when bradycardia occurs, the phenomenon is atropine responsive.5 Fasciculations are another known complication of SCh, but the clinical relevance of the fasciculations is debated.6,7 Malignant hyperthermia is a notorious but rare complication of SCh use. To date, no emergency department cases of malignant hyperthermia have been reported; the treatment for this disorder is cooling, sedation, and administration of dantrolene at 1 mg/kg IV.26
When SCh is contraindicated (Table 2), a nondepolarizing NMBA should be used for paralysis. Rocuronium is dosed at 1 mg/kg IV; the time of onset is 60 seconds, and duration of action is approximately 40-60 minutes.1 A Cochrane Review article comparing SCh and rocuronium showed that both agents provided clinically acceptable intubating conditions, but SCh was superior.27



One Response to “Rapid Sequence Intubation Pharmacology”
December 15, 2016
ravi singhHello Drs. Ahn and Solomon,
Thank you for a very nice summary. Are you able to provide a reference and guidance on ABW, IBW, LBW dosing for rocuronium, vecuronium, etomidate?
Regards,
Ravi Singh