
Much has changed in the realm of oral anticoagulation over the past few years. Beginning with the approval of dabigatran (Pradaxa), a direct thrombin inhibitor, patients received their first viable long-term alternative to warfarin. Subsequently, factor Xa inhibitors arrived on the market, initially featuring rivaroxaban (Xarelto) and apixaban (Eliquis). Now, edoxaban (Lixiana, Savaysa) has received FDA approval, and betrixaban is undergoing phase III trials. Experience with these classes of medications continues to increase, with several important new developments regarding safety and indications.
Explore This Issue
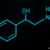
ACEP Now: Vol 34 – No 09 – September 2015Dabigatran
The most striking recent developments involve dabigatran. Dabigatran was initially hailed as a tremendous advance over warfarin, with a simple dosing schedule and lacking the requirement for ongoing laboratory monitoring of anticoagulant effect. However, despite its initial appeal, it was soon discovered that many patients were knowingly placed at untoward risk.1 Legal proceedings against Boehringer Ingelheim in the United States resulted in a $650 million settlement to compensate patients harmed by dabigatran, and just as important, discovery revealed an array of documents describing the manufacturer’s internal analyses.
While dabigatran was marketed as not requiring ongoing monitoring, the documents indicate Boehringer was aware that plasma levels of the drug were, in fact, quite variable depending on individual physiologic and genetic features. The internal analyses described fivefold variability in plasma levels, and dose adjustment based on plasma levels and monitoring could further reduce bleeding by 30 percent to 40 percent compared with warfarin. However, it was further determined that reporting the benefits of such testing “could result in a more complex message and a weaker value proposition.” Translation: maximizing profits trumps maximizing patient safety.
The importance of plasma level variability and monitoring is most evident when comparing the phase III trial populations with the target prescribing population. The pivotal RE-LY trial described the use of dabigatran in a population mostly younger than 75 years of age, but Boehringer’s marketing data indicate 30 percent of patients prescribed dabigatran are 80 years of age and older.2 The reduced renal excretion of the elderly results in supratherapeutic serum levels and unintended elevation of bleeding risk. Internal and regulatory approval documents reveal concerns regarding such risk that may have been specifically minimized by Boehringer representatives.
The bleeding risks associated with dabigatran have been of particular concern because, in contrast to warfarin or factor Xa inhibitors, there is no reliable pharmacologic reversal strategy. The only reported mechanism for reliable attenuation of its clinical effect has been hemodialysis. Now, such a development is on the horizon; an antibody fragment has reached phase III trials. Interim results from the RE-VERSE AD trial demonstrate rapid reduction of serum dabigatran levels following idarucizumab administration, although the pharmacologic effects seem to be durable only up to approximately 24 hours.3 Generally, this should be clinically adequate, however, as the half-life of dabigatran is typically 12 to 14 hours. The cost of such an antidote is likely to be quite high. Do you recall the more than $2,000 per vial price tag of CroFab? Frankly, the best strategy would simply be avoidance of dabigatran in the first place.
Andexanet Alfa and Aripazine/Ciraparantag
While prothrombin concentrate complexes appear to be viable reversal agents for the factor Xa inhibitors, development of alternative agents proceeds apace.4 Portola Pharmaceuticals, the same company pursuing development of betrixaban, is investigating andexanet alfa in phase III trials. Andexanet alfa is a recombinant factor Xa derivative with a higher affinity for the factor Xa inhibitors than native factor Xa. Therefore, circulating anticoagulants will preferentially bind andexanet alfa rather than native factor Xa. Substantially fewer data are currently available regarding this treatment than idarucizumab, but preliminary indications are favorable.
Pages: 1 2 3 | Single Page





No Responses to “New Developments in Direct Oral Anticoagulant Safety and Indications”