
Blujepa
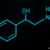
The least controversial of these new medications is likely Blujepa (gepotidacin), a straightforward new class of oral antibiotic. Blujepa, a triazaacenaphthylene antibiotic, inhibits DNA replication by blocking two different type II topoisomerase enzymes. In theory, with two sites of inhibition, the potential for gepotidacin resistance diminishes.
Explore This Issue
ACEP Now: August 2025 (Digital)The efficacy of Blujepa has been tested in the EAGLE series of clinical trials, with EAGLE-1 evaluating its efficacy against urogenital gonorrhea, and EAGLE-2 and EAGLE-3 against common pathogens causing uncomplicated urinary tract infections.9,10 EAGLE-1 compared oral treatment with Blujepa against ceftriaxone plus azithromycin, and both treatment regimens were similarly highly successful. The results from EAGLE-2 and EAGLE-3, testing Blujepa against nitrofurantoin are more complex, but need not be intricately dissected. Generally speaking, Blujepa was at least as efficacious as nitrofurantoin with respect to both symptom resolution and microbiological test of cure.
As with all new classes of antibiotics, appreciation for its introduction needs to be inversely proportional to enthusiasm for its use. The longer it is possible to hold off incorporating Blujepa into the typical treatment rotation, the longer it may be relied upon as a useful alternative. The side effect profile of Blujepa was generally benign. However, there was a clear increase in antibiotic-associated diarrhea, including infections with C. difficile.
The wholesale acquisition cost for Blujepa is also not yet available.
For now, it is unclear how much market penetration each of these drugs will have and how frequently they will be encountered by the typical emergency physician. However, whether by seeing them for potential adverse effects, after use for anaphylaxis, or unsolicited patient requests, it is worth being aware of these new options.
 Dr. Radecki (@EMLITOFNOTE) is an emergency physician and informatician with Christchurch Hospital in Christchurch, New Zealand. He is the Annals of Emergency Medicine podcast co-host and Journal Club editor.
Dr. Radecki (@EMLITOFNOTE) is an emergency physician and informatician with Christchurch Hospital in Christchurch, New Zealand. He is the Annals of Emergency Medicine podcast co-host and Journal Club editor.
References
- neffy. Highlights of Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/214697s000lbl.pdf Accessed May 7, 2025.
- Ellis AK, Casale TB, Kaliner M, et al. Development of neffy, an epinephrine nasal spray, for severe allergic reactions. Pharmaceutics. 2024;16(6):811.
- ARS Pharmaceuticals. SEC Form 8-K. 13 Jan 2025. Page 20.
- Jones J, Correll DJ, Lechner SM, et al. Selective inhibition of Na V 1.8 with VX-548 for acute pain. N Engl J Med. 2023;389(5):393-405.
- Vertex. Vertex Announces FDA Approval of JOURNAVX™ (suzetrigine), a First-in-Class Treatment for Adults With Moderate-to-Severe Acute Pain. https://news.vrtx.com/news-releases/news-release-details/vertex-announces-fda-approval-journavxtm-suzetrigine-first-class. Published January 30, 2025. Accessed May 7, 2025.
- Shrewsbury SB, Jeleva M, Satterly KH, et al. Stop 101: a phase 1, randomized, open‐label, comparative bioavailability study of inp104, dihydroergotamine mesylate (Dhe) administered intranasally by a i123 precision olfactory delivery (POD®) device, in healthy adult subjects. Headache. 2019;59(3):394-409.
- Albrecht D, Iwashima M, Dillon D, et al. A phase 1, randomized, open‐label, safety, tolerability, and comparative bioavailability study of intranasal dihydroergotamine powder (Sts101), intramuscular dihydroergotamine mesylate, and intranasal dhe mesylate spray in healthy adult subjects. Headache. 2020;60(4):701-712.
- Atzumi. Highlights of Prescribing Information. https://www.satsumarx.com/wp-content/uploads/2025/04/Atzumi-101-US-PI-1.pdf. Accessed May 7, 2025.
- Ross JDC, Wilson J, Workowski KA, et al. Oral gepotidacin for the treatment of uncomplicated urogenital gonorrhoea (EAGLE-1): a phase 3 randomised, open-label, non-inferiority, multicentre study. Lancet. 2025;405(10489):1608-1620.
- Wagenlehner F, Perry CR, Hooton TM, et al. Oral gepotidacin versus nitrofurantoin in patients with uncomplicated urinary tract infection (EAGLE-2 and EAGLE-3): two randomised, controlled, double-blind, double-dummy, phase 3, non-inferiority trials. Lancet. 2024;403(10428):741-755.
Pages: 1 2 3 4 | Single Page




No Responses to “What’s in The Drug Pipeline These Days?”