Explore This Issue
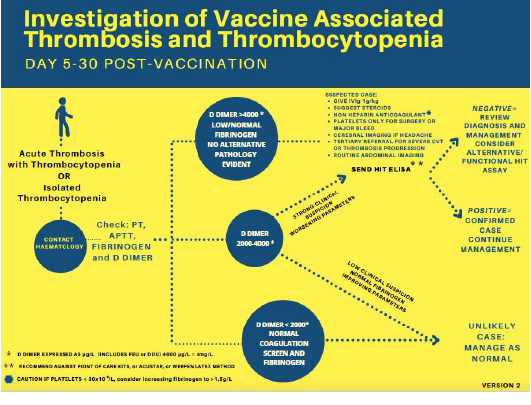
ACEP Now: Vol 40 – No 11 – November 2021Reproduced with permission from Pavord et al.9
Clinical Course and Resolution
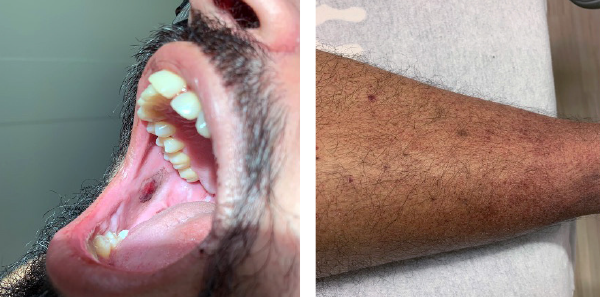
The patient was presumptively diagnosed with ITP, thought to be precipitated by the vaccine. He was immediately given intravenous immunoglobulin (IVIg) 1 gm/kg and methylprednisolone 60 mg IV. A CT scan of his brain was performed. A second tube of blood was sent to confirm the initial findings. The patient was promptly admitted to the ICU and underwent further workup, including HIV testing, flow cytometry of peripheral blood, hepatitis testing, antinuclear antibody (ANA) testing, and bone marrow biopsy with interventional radiology. ANA was positive at 1.4 (normal 0–0.9), and imaging revealed splenomegaly but unremarkable flow cytometry. The patient responded well to IVIg and high-dose IV steroids. He was subsequently discharged with oral prednisone after six days, with a platelet count of 285×10³/µL.
Discussion
While ITP has long been established in the medical literature, the novel COVID-19 viral infection and its respective vaccines, treatments, and side effects are still being studied across the globe. Both the illness itself and the remarkably effective vaccines have been associated with disruptions in the coagulation cascade.3 Discoveries and developments in these arenas are occurring almost daily. Currently, there are three COVID-19 vaccines approved by the Food and Drug Administration (FDA) for use in the United States: Pfizer-BioNtech, Moderna, and Johnson & Johnson.4–6
The Johnson & Johnson vaccine utilizes an adenovirus vector and is the only single-dose formulation.4–6 The Pfizer and Moderna formulations, by contrast, are each delivered in a two-shot regimen and have been heavily scrutinized as the first vaccines to employ the long-studied messenger RNA (mRNA) vector technology. In these vaccines, a genetically engineered mRNA molecule coding for the immunogenic coronavirus spike protein is encapsulated in a lipid nanoparticle, which facilitates cellular uptake, transport to ribosomes in the endoplasmic reticulum, and subsequent translation to endogenously produced spike protein.7 The mRNA is then rapidly degraded, decomposing within the cell, while the spike protein stimulates activated T cells to mount a protective immune response without the risk of active infection. While mRNA vaccine technology is relatively new in the world of vaccines, this method of treatment has been studied for years and successfully utilized in the treatment of certain cancers and genetic diseases.7,8
The pathogenesis of COVID-19 is complex and not fully understood. Thrombotic complications of the illness have been widely reported in patients with COVID-19, and early data suggest that the endogenous spike protein created by the vaccine response (whether by mRNA or adenovirus vector) could confer some risk of thrombotic and/or bleeding complications as well.9–13 One theory posits that the spike protein binds to the angiotensin-converting enzyme 2 receptors on endothelial cells, resulting in a pro-thrombotic cascade.9,10 It must be emphasized, however, that the data are conflicting and still emerging. Additionally, these effects were seen most compellingly with the AstraZeneca vaccine, which is not currently approved for use in the United States.8,9
Pages: 1 2 3 4 5 | Single Page




No Responses to “Rare But Emerging Conditions Associated with COVID-19 Vaccines”